Since their first synthesis in the 1950s, plastics are produced at a steadily increasing rate each year, with the vast majority of plastic being used for single-use, packaging purposes. Current mechanical recycling of plastic wastes downgrades the usefulness of plastic products, whilst chemical treatments often release harmful toxic products. The remaining plastic waste is either buried in landfill, where it may take centuries to degrade or enter the natural environment where it wreaks havoc at every level of ecosystems. In fact, if current plastic production and waste management practises continue, we will have dumped ~12,000 million tonnes of plastic waste into landfill and the environment by 20501. As well as curtailing our consumption of single-use plastics, we need to develop new ways to degrade plastic waste back into its original monomers for future use, reducing accumulation in the environment and limiting the production of plastic monomers from fossil fuel sources. In this white paper, we summarise the recent findings of insect larvae capable of ingesting and degrading plastics and we discuss how these discoveries could be applied for new, greener solutions to our plastic waste problem.

Table of Contents
The problem with plastics
Plastic polymers have been synthesised in mass since the 1950s and their production has increased exponentially ever since1. The vast majority of non-fibrous plastics are used for packaging because plastics can make flexible, durable, water-proof, and sterile containers in a diversity of shapes. However, the combination of single-uses of plastics and their recalcitrance to biodegradation has led to massive increases in plastics accumulating in municipal solid waste, which itself has increased in mass in the last 50 years. Plastics are also non-renewable as the hydrocarbon monomers for plastic polymer synthesis are derived from fossil fuels. There are three dominant plastic waste management options: (i) dumping plastics in landfill (or the natural environment) where they accumulate and degrade slowly over centuries, (ii) destroying plastics using thermal treatments that release toxic fumes, or (iii) mechanical recycling of plastics which produces poor quality plastic products (downcycling). Current estimations suggest that 6,300 million tonnes of plastic waste were generated by 2015, with only 9% recycled, 12% incinerated and 79% entering landfill or the natural environment1.
Needless to say that plastic waste accumulation in the environment wreaks havoc at all levels of ecosystems, including human health2. Much plastic waste in the environment is mechanically degraded into smaller microplastic particles over time by weathering and UV exposure from sunlight. Fibres from plastic textiles are also released as microplastics. Ingested microplastics have been found in all major animal groups, including humans, where they accumulate in the digestive systems, disrupt hormone systems, and inhibit reproduction. Marine life, in particular, is vulnerable to entanglement in macroplastics, leading to death. Microplastics also carry pollutants into the environment, especially waterways and the soil, and into food chains2. We desperately need a more efficient, cleaner recycling solution to both prevent the accumulation of further plastic waste in landfill and to remediate current plastic contamination of the natural environment.
Nature’s solution to plastic wastes
The most frequently-used plastics are considered non-biodegradable as they are highly recalcitrant to degradation in the natural environment, taking decades and sometimes centuries for soil microbes to degrade plastic fragments3. The fundamental problem with plastic waste is that the useful properties of plastic polymers (e.g. tough, waterproof, durable) also make them resist biodegradation by the usual degradative microbes3. Plastic polymers are long chains of hydrocarbon monomer units with strong, nonpolar carbon-carbon bonds and they form highly regular, crystalline structures that prevent the binding of hydrolytic enzymes4 (Figure 1). The hydrophobic, insoluble nature of plastics also hinders biodegradation by inhibiting colonisation and biofilm formation by degradative microbes4. Plastic polymers with larger surface areas, lower molecular weight, and less crystalline structure are relatively more amenable to enzymatic degradation3,5. Although some biodegradable plastics have been developed, they make up a limited proportion of the total produced plastics1 and so there remains a pressing need to find novel ways of degrading mass-produced plastics.
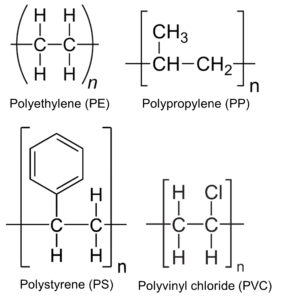
Figure 1. The chemical structures of four commonly-used plastic polymers. The repeated monomer unit is within brackets, where n represents the number of repeats.
Darkling beetle larvae
Despite the recalcitrance of plastics to biodegradation, nature may still offer a solution to our plastic waste problem. The ability of mealworms, the larvae of Darkling beetles (Tenebrionidae family), to ingest polystyrene has long been featured at high school science fairs6. Only recently have academic scientists started to test the ability of Darkling beetle larvae to ingest and degrade some of the most highly-produced plastics at a molecular level, including polyethylene (PE) (36% of total plastic production), polypropylene (PP) (21%), polyvinyl chloride (PVC) (12%), and polystyrene (PS) (<10%)1,7 (Fig. 1). An important experimental approach is gel permeation chromatography (GPC) analysis, which is used to characterise insect plastic depolymerization by separating degradation products from larval frass by size8. The parameters from GPC analysis are used to define broad (depolymerisation of all polymer molecules) versus limited (favoured depolymerisation of certain size polymer molecules) plastic depolymerisation9. The larvae of at least five Darkling beetle family have been shown to consume and degrade PS, including Tenebrio molitor (broad depolymerisation), Tenebrio obscurus (broad or limited), Tribolium castaneum (unknown), Zophobas atratus (broad or limited), and Plesiophthalmus davidis (broad)8,10-12 (Table 1). Larvae of T. molitor (broad and limited), T. obscurus (limited), and Z. atratus (limited) beetles are also able to degrade PE8-9,13-14. T. molitor (limited) and Z. atratus (limited) larvae can degrade PP15 and T. molitor larvae can further degrade PVC (limited) and rubber tire plastics8.
Pyralidae moth larvae
At the same time, larvae of pest moth species (waxworms, Pyralidae family), have also been shown to degrade PE including Plodia interpunctella, Galleria mellonella, and Achroia grisella16-18 (Table 1). There has been some argument over the experimental findings in Bombelli et al. (2017) reporting that homogenate from G. mellonella larvae is capable of degrading PE film19-20. The reported changes in chemical groups of PE molecules in the larval frass may have been due to organic contamination, and the mass loss of PE film could be due to repeated mechanical abrasion20. However, the ability of G. mellonella larvae to degrade PE once ingested has since been confirmed in further studies, which used appropriate controls including insect larvae that cannot degrade PE film21-23. G. mellonella larvae have also been reported to perform limited depolymerization of ingested PS23. Note, however, that some studies discuss the consumption of plastics by insect larvae without showing chemical analysis of frass to confirm the breakdown of polymer molecules – e.g. consumption of PP by G. mellonella larvae24.
Evolution and ecology
The ability of these insect larvae to variously degrade ingested plastics is due to evolved adaptations to their unusual ecological niches and corresponding diets. Many Darkling beetle species, including the Tenebrio genus, live in dead wood and feed on dropped leaves that contain lignin and cellulose7. Lignin and cellulose are both hydrophobic, organic polymers that are relatively recalcitrant to enzymatic degradation meaning that Darkling beetles possess specialised adaptations to digest these compounds7. Both the greater waxworm (G. mellonella) and the lesser waxworm (A. grisella) are parasites of beehives that feed on beeswax16. Beeswax is made up of a mix of long-chain aliphatic alcohols and hydrocarbons, meaning that these waxworm species have evolved to degrade similar hydrocarbon compounds to plastic polymers, particularly PE17,25. As we start to characterise the plastic-degradation abilities of these insect larvae, we are only beginning to understand how they degrade plastic polymers and how these findings might translate to solutions to our plastic waste problems17.
Table 1. Insect species with larvae capable of degrading plastics. Rates of plastic consumption are reported as a range from the referenced literature. PS, polystyrene; PE, polyethylene; PP, polypropylene.
| Species name | Phylum and order | PS consumption? | PE consumption? | PP consumption? | Reproduction on plastic? | References |
| Tenebrio molitor (yellow mealworm) | Arthropoda, Coleoptera | Yes 0.12-0.27 mg/d per larva | Yes 0.05-0.23 mg/d per larva | Yes 0.01 mg/d per larva | Yes, on PS alone | 6, 9, 12, 14 |
| Tenebrio obscurus (dark mealworm) | Arthropoda, Coleoptera | Yes 0.32 mg/d per larva | Yes 0.034-0.045 mg/d per larva | Unknown | Not reported | 9, 26 |
| Tribolium castaneum (red flour beetle) | Arthropoda, Coleoptera | Yes Rate not reported | Unknown | Unknown | Not reported | 27 |
| Zophobas atratus (superworm) | Arthropoda, Coleoptera | Yes 0.58-1.41 mg/d per larva | Yes 0.30-0.59 mg/d per larva | Yes 0.031 mg/d per larva | Yes, on PS alone and PE with bran | 11, 13, 15, 28 |
| Plesiophthalmus davidis | Arthropoda, Coleoptera | Yes 2.44 mg/d per larva | Unknown | Unknown | Not reported | 10 |
| Plodia interpunctella (Indian mealmoths) | Arthropoda, Lepidoptera | Unknown | Yes Rate not reported | Unknown | Not reported | 18 |
| Galleria mellonella (greater waxworm) | Arthropoda, Lepidoptera | Yes 0.10-0.28 mg/d per larva | Yes 0.02-0.04 mg/d per larva | Yes 0.008 mg/d per larva | Yes, on PS, PE or PP alone | 17, 23-24 |
| Achroia grisella (lesser waxworm) | Arthropoda, Lepidoptera | Unknown | Yes 1.83 mg/day per larva | Unknown | Yes, on PE alone | 16 |
How do insect larvae degrade plastic polymers?
Contributions from the microbiome
One obvious explanation of how insect larvae degrade ingested plastics is by harnessing microbes hosted in their gut, in an analogous way to microbe-dependent degradation of lignocellulose in the hindgut of termites. Microbes inhabiting their guts may express enzymes capable of degrading PS and PE polymers4,29. The first stage of microbial plastic degradation is biodeterioration, the formation of a biofilm on the surface of ingested plastic fragments. The next stage, biofragmentation, sees microbial enzymes acting on the plastic polymers including the incorporation of oxygen into the carbon chain and, later, the transformation of polymer side chains. Smaller degradation products may be transported across the cell membrane of the gut microbes, entering the microbial cytoplasm for assimilation. During the final mineralisation stage, the degradation products undergo metabolic enzymatic reactions, particularly those of the Krebs cycle and lipid synthesis, releasing oxidised metabolites including carbon dioxide, methane, and water4.
The first evidence of microbe-dependent degradation of plastics by insect larvae came from antibiotic suppression assays, where the impact of feeding the larvae antibiotics on biodegradation is monitored by chemical assays. In particular, biodegradation of PS, PVC, and PP seems to be reliant on gut microbiota. Feeding gentamicin to T. molitor larvae prevented them from releasing carbon dioxide from PS12,30, which was also confirmed for T. obscurus26,31. A mixture of antibiotics also reduced the ability of Z. atratus larvae to degrade PS11,28. Similarly, gentamicin treatment nearly completely arrested PVC degradation by T. molitor larvae8 and largely eliminated PP depolymerisation by T. molitor and Z. atratus larvae15. The antibiotic assays suggest that insect larvae are less reliant on their gut microbiome for PE degradation. For example, gentamicin treatment did not prevent PE degradation by T. molitor and T. obscurus9,30, and only halved glycol excretion of G. mellonella feeding on PE22. In contrast, gentamicin almost completely prevented PE degradation by Z. atratus larvae28. In summary, antibiotics prevent PS degradation but only partially inhibit PE degradation, suggesting there are host factors (e.g. gut enzymes) involved in PE degradation. The involvement of host factors may explain why PE depolymerization tends to be limited, as insect-expressed enzymes may only be capable of degrading polymer molecules of specific lengths.
The next stage of the investigation of the insect larval gut microbiome involved identifying and testing microbes from the gut for their ability to degrade solid PS and/or PE film in vitro. A number of PS-degrading bacterial strains have been identified using RNA sequencing of the gut microbiome. For example, Exiguobacterium spp. strain YT2 from the T. molitor gut12, Acinetobacter spp. AnTc-1 from the T. castaneum gut27 and Serratia spp. from the Plesiophthalmus davidis gut10 were capable of degrading PS film over many days of incubation. Other sequencing studies identified various Citrobacter, Serratia and Enterobacter bacterial spp. associated with PS and PP diets in the guts of T. molitor and Z. atratus larvae13,15,28,31. Interestingly, such microbiome sequencing has also identified bacterial strains capable of PE degradation, despite the antibiotic suppression assays showing a limited involvement for microbes in PE degradation. Acinetobacter spp. from the gut of G. mellonella larvae22, and Enterobacter abscuriae YT1 and Bacillus spp. YP1 from the gut of P. interpunctella18, were capable of degrading PE film when incubated for several months to one year. Citrobacter bacterial spp. was also strongly associated with PE degradation in the gut microbiota of T. molitor larvae14 and that of Z. atratus larvae13.
These microbiome studies have repeatedly identified bacterial species in the Enterobacteriaceae family (particularly Serratia and Citrobacter genus), and members of the Acinetobacter and Bacillus genus, capable of degrading PS and PE plastics suggesting that these may have general plastic-degrading abilities and/or interact to metabolise the polymers. Note, however, that few of these studies actually confirmed that the bacteria associated with plastic diets were capable of plastic degradation in vitro. Future screens should systematically test the ability of bacterial species related to these groups to degrade a range of plastic substrates in vitro, both as single strains and when co-cultured (to test for synergistic interactions).
New players: salivary secretions and host enzymes
As mentioned, antibiotic treatment does not always completely inhibit PE degradation in these insect larvae, suggesting that there are endogenous host factors also required for extensive plastic degradation31. Recent studies are beginning to elucidate these host factors. For example, fractionation of T. molitor larvae gut supernatant identified a host-produced emulsifying factor that increases PS bioavailability and improves larval rates of respiration on a PS diet31. An important recent preprint reports a proteomic assessment of the saliva of G. mellonella larvae fed PE, identifying an arylphorin enzyme (designated Demetra) and a hexamerin enzyme (designated Ceres) that are capable of oxidising the surface of PE film21. Although both Demetra and Ceres enzymes are related to protein families with oxidase activity, it is unclear how these particular salivary enzymes degrade PE21. Future work will inevitably investigate both the mechanism of these enzymes and assess the ability of related enzymes to act on PS polymers. More generally, transcriptomic and proteomic studies of sterile salivary and gut secretions from these insect larvae, comparing gene expression between plastic- and bran-fed larvae, will identify other host factors involved in plastic biodegradation.
A working model
Combining our understanding of the gut microbiome and host-produced enzymes, Yang et al. (2015) proposed a model of plastic degradation in the gut of Darkling beetle larvae12:
- Plastics are chewed into smaller particles, and exposed to salivary enzymes, increasing the surface area for further digestion by bacterial and host-produced enzymes.
- Inhaled air brings oxygen into the insect intestine which acts as an electron acceptor for aerobic microbial metabolism.
- The ingested plastic is further reduced to smaller fragments by mechanical movements of the gut walls, further increasing the surface area for enzymatic degradation.
- Some of the plastic polymer derivatives pass into host cells lining the gut and gut microbes for metabolism into carbon dioxide, methane, and water.
- Some of the derivatives and undigested plastic are excreted by the larva as frass.
Scale-up for industry
As our working model of plastic degradation by insect larvae develops, we propose that there are solutions to sustainable and efficient chemical management of plastic wastes (Fig. 2).
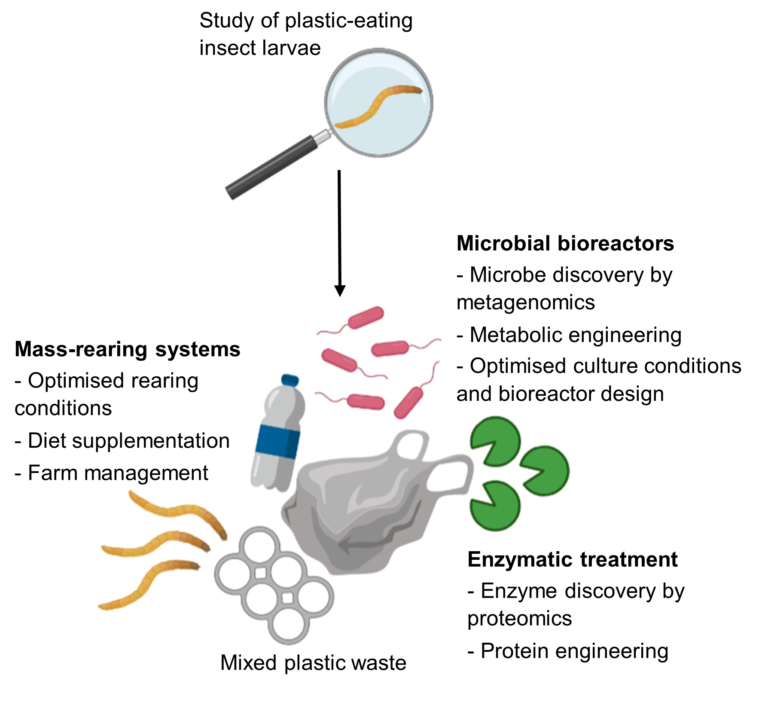
Figure 2. The study of plastic-feeding insect larvae offers potential solutions for the biodegradation of mixed plastic wastes.
Mass insect rearing
Culturing insect larvae at a large scale has been investigated as a solution to mass produce larvae for pest population management, by the release of parasitoids and sterilised pest species, as well as for sustainable food production32-33. The same insect mass-rearing systems could be applied to culturing insect larvae on plastic waste for biodegradation. The species of insect larvae selected for plastic waste degradation (Table 1) will determine several factors influencing the optimised feeding and reproductive rates of the larvae, including temperature, rearing density, and food substrates33. Recently, a French project called DESIgning the Insect Biorefinery to Contribute to a More Sustainable Agro-Food Industry (DESIREABLE) investigated the management of farms mass-culturing T. molitor larvae to be trialled as a sustainable food source32. In these farms, multilevel rearing boxes with different mesh sizes were used to separate instars of larvae and reproductive adults34. Breeding and fattening T. molitor populations were separated, but this could be amended to maximise feeding larvae on plastic waste substrate whilst keeping a mixed reproductive group to maintain the farm population size. Management scenarios, timings, and workforce requirements were also measured for differing size farms34. Whilst the rate of organic substrate degradation by T. molitor was not reported, the DESIREABLE project offers valuable information for scaling up T. molitor larval culturing for plastic waste biodegradation.
A recent study performed a life cycle/techno-economic analysis of mass-rearing insect larvae for PE degradation to release glycol for biofuel production19. The authors concluded that even at optimistic rates of PE degradation, the net treatment cost would be over 300 euros and 4-10 tonnes of T. molitor larvae per tonne of PE plastics. The mass-rearing approach would have a high carbon footprint to maintain the optimum climate for rearing insect larvae, and this is likely to generate PE flakes and microplastics that would prevent using these insects in the human food chain19. However, the authors did not consider an intrinsic environmental-economic benefit of removing PE waste without generating toxic by-products.
Nutritional supplementation
Billen et al. (2020) also did not include combining different organic waste streams to improve the efficiency of plastic degradation by insect larvae19. Nutritional supplementation of plastic waste with organic waste could boost plastic degradation rates by providing the larvae with nutrients (especially nitrogen) that are not found in the hydrocarbon plastic polymers35. For T. molitor larvae, dietary supplementation with wheat bran, corn flour or soy protein improved PS consumption and larval survival rates, and increased biomass gain on a mixed plastics diet6,14,26. Similarly, adding bran to a diet of PVC partially restored the survival rates of T. molitor larvae28. In contrast, supplementing the beeswax diet of G. mellonella larvae with bran increased larval survival rates but not consumption rates of PS and PE23. A. grisella larvae generally showed high consumption and survival rates when feeding on a diet of PE and bran16. Different organic substrates can impact larval growth in a manner that does not scale linearly from benchtop to industrial scale36, so we will need comparative studies of organic dietary supplements for rearing plastic-degrading insect species.
Selective breeding
Despite the limited nutrients available in hydrocarbon plastic polymers, some of the plastic-feeding insect species (T. molitor, Z. atratus, and A. grisella) are reportedly able to complete their lifecycle and reproduce on plastic-only diets, opening the way to selectively breeding these species to enhance their survival and degradation of PS and PE wastes6,11,16 (Table 1). In particular, the second generation of T. molitor larvae raised on a PS diet maintained similar PS degradation rates to the first generation, demonstrating that selective breeding would be feasible for T. molitor6. The same result was found for PE degradation rates by A. grisella larvae16. Insect larvae may be particularly amenable to selective breeding as they have short generation times, and relatively large populations can be maintained in a small container which is important to avoid reduced fecundity from inbreeding depression37.
Microbial culture
The analysis of using insect larvae for PE degradation concluded that it would be economically feasible to use these insects to discover the microbes and/or enzymes involved in plastic degradation for application at scale19.
Bioreactors
Both pure and mixed microbe cultures have been used for mass processing of different types of waste using established bioreactor designs38. Landfill bioreactors contrast the conventional dry-tomb landfill approach in encouraging microbial growth for aerobic or anaerobic degradation of municipal solid waste by circulating leachate, providing moisture and nutrients for the microbes. Landfill reactors may be more efficient than dry landfill at degrading organic solid wastes but require additional management such as mechanical processing of waste, nutrient addition, and pH and temperature management. It is also important to optimise the oxygen supply to microbial communities in the bioreactor, especially because the degradation of plastics requires thermos-oxidative reactions39-40. Alternatively, immobilized and membrane-based bioreactors could be used to clear wastewater of plastic particles41-42. Immobilization of microbes onto matrices improves the efficiency of removal of contaminants from wastewater flowing over the matrices43. There are a number of other bioreactor designs for bioremediation of organic contaminants from wastewater, such as stirred-tank bioreactors, biofilters, and bioscrubbers44.
Microbial consortia
Currently, the main limitation of using microbial culture for plastic degradation is our poor understanding of how microbes synergistically contribute to the degradation of plastics within the insect gut, and how we can improve the efficiency when culturing microbes in vitro. Disappointingly low rates of plastic degradation have been reported from single cultures identified from insect larvae guts. The bacterium Bacillus spp. YP1 caused a ~10% mass decrease of a PE film over 60 days of incubation18. Similarly, Exiguobacterium spp. strain YT2 degraded ~7.5% of the mass of PS pieces over a 60-day incubation12. An Acinetobacter spp., designated AnTc-1, was slightly more efficient at degrading PS with a ~12% decrease in PS mass over the 60-day incubation27.
Microbial consortia isolated from insect larval guts are likely to be more efficient for plastic degradation because complete degradation often requires synergistic interactions (symbiosis) between multiple microbial species4,10. An interesting approach by Brandon et al. (2021) developed microbial enrichments on PS film following original inoculation from the gut microbiome of T. molitor larvae31. The microbial enrichment was more efficient at PS degradation than freshly extracted gut microbiota from PS-fed T. molitor, suggesting that this enrichment approach could be used to select for PS-degrading gut microbes. However, the authors were unable to produce an enrichment that degrades PS film more rapidly than T. molitor larvae themselves, demonstrating that we are far from being able to replicate the gut microbiome for biodegradation31. Future studies will use ‘omics technology, especially metagenomics, to identify further plastic-degrading microbes from insect larvae guts and start to elucidate how metabolic interactions between gut microbes lead to complete polymer degradation4. At that point, the efficiency of degradation could be improved by using synthetic biology to engineer these metabolic pathways into a single model microbe that is more amenable to mass culture in bioreactors4.
Enzymatic treatment
Once the host-produced and microbial enzymes responsible for plastic degradation have been identified, it may be more efficient to directly apply enzyme treatments to plastic waste45. As with microbial bioreactors, systems for treating municipal solid waste with enzymes are already established – e.g. for the degradation of lignocellulose by peroxidase enzymes46. Enzymatic treatments are more easily controlled than applying microbial consortia, avoiding potential issues with contamination, and reducing the risk of producing toxic by-products. If complete enzymatic degradation of plastic polymers could be achieved, enzymatic treatments would prevent downcycling plastic waste because the released monomers could be valuable for future plastic production47. In the realm of plastic waste recycling, enzymatic treatments are currently being developed for the degradation of polyethylene terephthalate (PET) polymers48. This approach is based on the discovery of an efficient bacterial PETase and subsequent studies of factors impacting its activity, such as PET crystallinity, temperature, and pH49. The EU Horizon 2020 project MIXed Plastics Biodegradation and Upcycling using Microbial Communities (Mix-Up) aims to combine such enzymatic treatments with mixed culture bioreactors to establish a circular economy for plastic waste recycling47.
As previously described, ‘omics technology will help to establish a complete pathway of enzymatic degradation of common plastic polymers50. For example, metagenomics of plastic-enriched environments, and proteomic studies of known plastic-degrading microbes, will contribute to enzyme discovery. Synthetic biology will then be critical for improving enzymes and making them better suited to the enzymatic treatment of municipal solid waste50. For example, one group successfully used machine learning to predict amino acid substitutions that improve the thermostability and activity of the PETase used to degrade PET51. Protein engineering can further improve the binding of polymer substrates to the active site and alter the specificity of the plastic-degrading enzymes, opening the field to mixed plastic waste recycling50.
Conclusions
The academic investigation into the molecular mechanisms by which insect larvae degrade particular plastic polymers has only just begun, and we have argued that this has already generated insights into how we might deal with our plastic waste in a clean, sustainable manner. The potential plastic waste solutions are possible at three informational levels, from the least information required to the most: mass insect rearing, microbial bioreactors, and enzymatic treatments (Fig. 2). The latter two solutions are not necessarily distinct, with microbe discovery leading to enzyme discovery and vice. versa.
The long-term goal, as outlined by the EU Horizon Mix-Up project, is towards applying mixed microbial cultures and specific enzyme treatments to mixed plastic wastes in order to regenerate plastic monomers – i.e. a circular economy for plastic waste. Systems biology, particularly large-scale ‘omics projects, will lead to the discovery of more plastic-degrading microbes and their enzymes from the guts of insect larvae. Subsequently, synthetic biology will optimise plastic degradation by microbes and enzymes such as by engineering metabolic pathways and enzyme engineering.
References
- Geyer, R., Jambeck, JR. and Law, KL. (2017) Production, use, and fate of all plastics ever made. Science. [Online] 3(7), e1700782. doi: 10.1126/sciadv.1700782
- Li, WC., Tse, HF. and Fok, L. (2016) Plastic waste in the marine environment: A review of sources, occurrence and effects. Science of the Total Environment. [Online] 566-567, 333-349. doi: 10.1016/j.scitotenv.2016.05.084
- Ahmed, T., Shahid, M., Azeem, F., Rasul, I., Shah, AA., Noman, M., Hameed, A., Manzoor, N., Manzoor, I. and Muhammed, S. (2018) Biodegradation of plastics: current scenario and future prospects for environmental safety. Environmental Science and Pollution Research. [Online] 25(8), 7287-7298. doi: 10.1007/s11356-018-1234-9
- Jaiswal, S., Sharma, B. and Shukla, P. (2020) Integrated approaches in microbial degradation of plastics. Environmental Technology and Innovation. [Online] 17, 100567. doi: 10.1016/j.eti.2019.100567
- Tokiwa, Y., Calabia, BP., Ugwu, CU. and Aiba, S. (2009) Biodegradability of plastics. International Journal of Molecular Science. [Online] 10(9), 3722-3742. doi: 10.3390/ijms10093722
- Yang, S-S., Brandon, AM., Flanagan, JCA., Yang, J., Ning, D., Cai, S-Y., Fan, H-Q., Wang, Z-Y., Ren, J., Benbow, E., Ren, N-Q., Waymouth, RM., Zhou, J., Criddle, CS. and Wu, W-M. (2018) Biodegradation of polystyrene wastes in yellow mealworms (larvae of Tenebrio molitor Linnaeus): Factors affecting biodegradation rates and the ability of polystyrene-fed larvae to complete their life cycle. Chemosphere. [Online] 191, 979-989. doi: 10.1016/j.chemosphere.2017.10.117
- Wu, W-M. and Criddle, CS. (2021) Characterization of biodegradation of plastics in insect larvae. Methods in Enzymology. [Online] 648, 95-120. doi: 10.1016/bs.mie.2020.12.029
- Peng, B-Y., Chen, Z., Chen, J., Yu, H., Zhou, X., Criddle, CS., Wu, W-M. and Zhang, Y. (2020) Biodegradation of polyvinyl chloride (PVC) in Tenebrio molitor (Coleoptera: Tenebrionidae) larvae. Environment International. [Online] 145, 106106. doi: 10.1016/j.envint.2020.106106
- Yang, S-S., Ding, M-Q., Zhang, Z-R., Ding, J., Bai, S-W., Cao, G-L., Zhao, L., Pang, J-W., Xing, D-F., Ren, N-Q. and Wu, W-M. (2021a) Confirmation of biodegradation of low-density polyethylene in dark- versus yellow-mealworms (larvae of Tenebrio obscurus versus Tenebrio molitor) via gut microbe-independent depolymerization. Science of the Total Environment. [Online] 789, 147915. doi: 10.1016/j.scitotenv.2021.147915
- Woo, S., Song, I. and Cha, HJ. (2020) Fast and facile biodegradation of polystyrene by the gut microbial flora of Plesiophthalmus davidis larvae. Applied Environmental Microbiology. [Online] 86(18), e01361-20. doi: 10.1128/AEM.01361-20
- Yang, Y., Wang, J. and Xia, M. (2020) Biodegradation and mineralization of polystyrene by plastic-eating superworms Zophobas atratus. Science of the Total Environment. [Online] 708, 135233. doi: 10.1016/j.scitotenv.2019.135233
- Yang, Y., Yang, J., Wu, W-M., Zhao, J., Song, Y., Gao, L., Yang, R. and Jiang, L. (2015) Biodegradation and mineralization of polystyrene by plastic-eating mealworms: part 2. Role of gut microorganisms. Environmental Science and Technology. [Online] 49(20), 12087-12093. doi: 10.1021/acs.est.5b02663
- Luo, L., Wang, Y., Guo, H., Yang, Y., Qi, N., Zhao, X., Gao, S. and Zhou, A. (2021) Biodegradation of foam plastics by Zophobas atratus larvae (Coleoptera: Tenebrionidae) associated with changes of gut digestive enzyme activities and microbiome. Chemosphere. [Online] 282, 131006. doi: 10.1016/j.chemosphere.2021.131006
- Brandon, AM., Gao, S-H., Tian, R., Ning, D., Yang, S-S., Zhou, J., Wu, W-M. and Criddle, CS. (2018) Biodegradation of polyethylene and plastic mixtures in mealworms (larvae of Tenebrio molitor) and effects on the gut microbiome. Environmental Science and Technology. [Online] 52(11), 6526-6533. doi: 10.1021/acs.est.8b02301
- Yang, S-S., Ding, M-Q., Zhang, C-H., Li, Q-X., Xing, D-F., Cao, G-L., Zhao, L., Ding, J., Ren, N-Q. and Wu, W-M. (2021b) Biodegradation of polypropylene by yellow mealworms (Tenebrio molitor) and superworms (Zophobas atratus) via gut-microbe-dependent depolymerization. Science of the Total Environment. [Online] 756, 144087. doi: 10.1016/j.scitotenv.2020.144087
- Kundungal, H., Gangarapu, M., Sarangapani, S., Patchaiyappan, A. and Devipriya, SP. (2019) Efficient biodegradation of polyethylene (HDPE) waste by the plastic-eating lesser waxworm (Achroia grisella). Environmental Science and Pollution Research. [Online] 26, 18509-18519. doi: 10.1007/s11356-019-05038-9
- Bombelli, P., Howe, CJ. and Bertocchini, F. (2017) Polyethylene bio-degradation by caterpillars of the wax moth Galleria mellonella. Current Biology. [Online] 27(8), 292-293. doi: 10.1016/j.cub.2017.02.060
- Yang, J., Yang, Y., Wu, W-M., Zhao, J. and Jiang, L. (2014) Evidence of polyethylene biodegradation by bacterial strains from the guts of plastic-eating waxworms. Environmental Science and Technology. [Online] 48(23), 13776-13784. doi: 10.1021/es504038a
- Billen, P., Khalifa, L., van Gerven, F., Tavernier, S. and Spatari, S. (2020) Technological application potential of polyethylene and polystyrene biodegradation by macro-organisms such as mealworms and wax moth larvae. Science of the Total Environment. [Online] 735, 139521. doi: 10.1016/j.scitotenv.2020.139521
- Weber, C., Pusch, S. and Opatz, T. (2017) Polyethylene bio-degradation by caterpillars? Current Biology. [Online] 27(15), 744-745. doi: 10.1016/j.cub.2017.07.004
- Sanluis-Verdes, A., Colomer-Vidal, P., Rodriguez-Ventura, F., Bello-Villarino, M., Spinola-Amilibia, M., Ruiz-Lopez, E., Illanes-Vicioso, R., Castroviejo, P., Aiese Cigliano, R., Montoya, M., Falabella, P., Pesquera, C., Gonzalez-Legarreta, L., Arias-Palomo, E., Sola, M., Torroba, T., Arias, CF. and Bertocchini, F. (2022, preprint) Wax worm saliva and the enzymes therein are the key to polyethylene degradation by Galleria mellonella. bioRxiv. [Online] doi: 10.1101/2022.04.08.487620
- Cassone, BJ., Grove, HC., Elebute, O., Villanueva, SMP. and LeMoine, CMR. (2020) Role of intestinal microbiome in low-density polyethylene degradation by caterpillar larvae of the greater wax moth, Galleria mellonella. Proceedings of the Royal Society B: Biological Sciences. [Online] 287, 20200122. doi: 10.1098/rspb.2020.0112
- Lou, Y., Ekaterina, P., Yang, S-S., Lu, B., Liu, B., Ren, N., Corvini, PF-X. and Xing, D. (2020) Biodegradation of polyethylene and polystyrene by greater wax moth larvae (Galleria mellonella L.) and the effect of co-diet supplementation on the core gut microbiome. Environmental Science and Technology. [Online] 54(5), 2821-2831. doi: 10.1021/acs.est.9b07044
- Barrionuevo, RJM., Martin, E., Galindo CA., Malizia, A., Chalup, A., de Cristobal, RE. and Monmany Garzia, AC. (2022) Consumption of low-density polyethylene, polypropylene, and polystyrene materials by larvae of the greater wax moth, Galleria mellonella L. (Lepidoptera, Pyralidae), impacts on their ontogeny. Environmental Science and Pollution Research International. [Online] doi: 10.1007/s11356-022-20534-1
- Kong, HG., Kim, HH., Chung, J-H., Jun, J., Lee, S., Kim, H-M., Jeon, S., Park, SG., Bhak, J. and Ryu, C-M. (2019) The Galleria mellonella hologenome supports microbiota-independent metabolism of long-chain hydrocarbon beeswax. Cell Reports. [Online] 26(9), 2451-2464. doi: 10.1016/j.celrep.2019.02.018
- Peng, B-Y., Su, Y., Chen, Z., Chen, J., Zhou, X., Benbow, ME., Criddle, CS., Wu, W-M. and Zhang, Y. (2019) Biodegradation of polystyrene by dark (Tenebrio obscurus) and yellow (Tenebrio molitor) mealworms (Coleoptera: Tenebionidae). Environmental Science and Technology. [Online] 53(9), 5256-5265. doi: 10.1021/acs.est.8b06963
- Wang, Z., Xin, X., Shi, X. and Zhang, Y. (2020) A polystyrene-degrading Acinetobacter bacterium isolated from the larvae of Tribolium castaneum. Science of the Total Environment. [Online] 726, 138564. doi: 10.1016/j.scitotenv.2020.138564
- Peng, B-Y., Li, Y., Fan, R., Chen, Z., Chen, J., Brandon, AM., Criddle, CS., Zhang, Y. and Wu, W-M. (2020) Biodegradation of low-density polyethylene and polystyrene in superworms, larvae of Zophobas atratus (Coleoptera: Tenebrionidae): Broad and limited extent depolymerization. Environmental Pollution. [Online] 266, 155206. doi: 10.1016/j.envpol.2020.115206
- Amobonye, A., Bhagwat, P., Singh, S. and Pilai, S. (2021) Plastic biodegradation: Frontline microbes and their enzymes. Science of the Total Environment. [Online] 759, 143536. doi: 10.1016/j.scitotenv.2020.143536
- Yang, L., Gao, J., Liu, Y., Zhuang, G., Peng, X., Wu, W-M. and Zhuang, X. (2021c) Biodegradation of expanded polyethylene foams in larvae of Tenebrio molitor Linnaeus (Coleoptera: Tenebrionidae): Broad versus limited extent depolymerization and microbe-dependence versus independence. Chemosphere. [Online] 262, 127818. doi: 10.1016/j.chemosphere.2020.127818
- Brandon, AM., Garcia, AM., Khlystov, NA., Wu, W-M. and Criddle, CS. (2021) Enhanced bioavailability and microbial biodegradation of polystyrene in an enrichment derived from the gut microbiome of Tenebrio molitor (mealworm larvae). Environmental Science and Technology. [Online] 55(3), 2027-2036. doi: 10.1021/acs.est.0c04952
- Cadinu, LA., Barra, P., Torre, F., Delogu, F. and Madau, FA. (2020) Insect rearing: Potential, challenges, and circularity. Sustainability. [Online] 12(11), 4567. doi: 10.3390/su12114567
- Sorensen, JG., Addison, MF. and Terblanche, JS. (2012) Mass-rearing of insects for pest management: Challenges, synergies and advances from evolutionary physiology. Crop Protection. [Online] 38, 87-94. doi: 10.1016/j.cropro.2012.03.023
- Maillard, F., Macombe, C., Aubin, J., Romdhana, H. and Mezdour, S. (2018) ‘Mealworm larvae production systems: management scenarios’ in Halloran, A., Flore, R., Vantomme, P. and Roos, N. (eds.) Edible Insects in Sustainable Food Systems. Springer: Switzerland, pp. 277-301.
- Yang, S. and Wu, W. (2020) ‘Biodegradation of plastics in Tenebrio genus (mealworms)’ in He, D. and Luo, L. (eds.) Microplastics in Terrestrial Environments. Springer Nature: Switzerland, pp. 385-422.
- Scala, A., Cammack, JA., Salvia, R., Scieuzo, C., Franco, A., Bufo, SA., Tomberlin, JK. and Falabella, P. (2020) Rearing substrate impacts growth and macronutrient composition of Hermetia illucens (L.) (Diptera: Stratiomyidae) larvae produced at an industrial scale. Scientific Reports. [Online] 10, 19448. doi: 10.1038/s41598-020-76571-8
- Kuriwada, T., Kumano, N., Shiromoto, K. and Haraguchi, D. (2010) Effect of mass rearing on life history traits and inbreeding depression in the sweetpotato weevil (Coleoptera: Brentidae). Journal of Economic Entomology. [Online] 103(4), 1144-1148. doi: 10.1603/EC09361
- Kumar, S., Chiemchaisri, C. and Mudhoo, A. (2011) Bioreactor landfill technology in municipal solid waste treatment: an overview. Critical Reviews in Biotechnology. [Online] 31(1), 77-97. doi: 10.3109/07388551.2010.492206
- Ghosh, SK., Pal, S. and Ray, S. (2013) Study of microbes having potentiality for biodegradation of plastics. Environmental Science and Pollution Research. [Online] 20, 4339-4355. doi: 10.1007/s11356-013-1706-x
- Garcia-Ochoa, F. and Gomez, E. (2009) Bioreactor scale-up and oxygen transfer rate in microbial processes: An overview. Biotechnology Advances. [Online] 27(2), 153-176. doi: 10.1016/j.biotechadv.2008.10.006
- Zhou, Y., Kumar, M., Sarsaiya, S., Sirohi, R., Awasthi, SK., Sindhu, R., Binod, P., Pandey, A., Bolan, NS., Zhang, Z., Singh, L., Kumar, S. and Awasthi, MK. (2022) Challenges and opportunities in bioremediation of micro-nano plastics: A review. Science of the Total Environment. [Online] 802, 149823. doi: 10.1016/j.scitotenv.2021.149823
- Caruso (2015) Plastic degrading microorganisms as a tool for bioremediation of plastic contamination in aquatic environments. Journal of Pollution Effects and Control. [Online] 3(3), 100e112. doi: 10.4172/2375-4397.1000e112
- Portier, RJ. and Miller, GP. (1991) Immobilized microbe bioreactors for waste water treatment. Waste Management and Research. [Online] 9(5), 445-451. doi: 10.1016/0734-242X(91)90075-I
- Srivastava, AK., Singh, RK. and Singh, D. (2021) ‘Microbe-based bioreactor system for bioremediation of organic contaminants: present and future perspective’ in Kumar, A., Singh, VK., Singh, P. and Mishra, VK. (eds.) in Microbe Mediated Remediation of Environmental Contaminants. Woodhead Publishing: Sawston, UK, pp. 241-253.
- Karam, J. and Nicell, JA. (1999) Potential applications of enzymes in waste treatment. Journal of Chemical Technology and Biotechnology. [Online] 69(2), 141-153. doi: 10.1002/(SICI)1097-4660(199706)69:2<141::AID-JCTB694>3.0.CO;2-U
- Jayasinghe, PA., Hettiaratchi, JPA., Mehrota, AK. and Kumar, S. (2011) Effect of enzyme additions on methane production and lignin degradation of landfilled sample of municipal solid waste. Bioresource Technology. [Online] 102(7), 4633-4637. doi: 10.1016/j.biortech.2011.01.013
- Ballerstedt, H., Tiso, T., Wierckx, N., Wei, R., Averous, L., Bornscheuer, U., O’Connor, K., Floehr, T., Jupke, A., Klankermayer, J., Liu, L., de Lorenzo, V., Narancic, T., Nogales, J., Perrin, R., Pollet, E., Prieto, A., Casey, W., Haarmann, T., Sarbu, A., Schwaneberg, U., Xin, F., Dong, W., Xing, J., Chen, G-Q., Tan, T., Jiang, M. and Blank, LM. (2021) MIXed plastics biodegradation and UPcycling using microbial communities: EU Horizon 2020 project MIX-UP started January 2020. Environmental Sciences Europe. [Onlin] 33, 99. doi: 10.1186/s12302-021-00536-5
- Maurya, A., Bhattacharya, A. and Khare, SK. (2020) Enzymatic remediation of polyethylene terephthalate (PET)-based polymers for effective management of plastic wastes: an overview. Bioprocess Engineering. [Online] 8, 602325. doi: 10.3389/fbioe.2020.602325
- Cornwall, W. (2021) The plastic eaters. Science. [Online] 373(6650), 36-39. doi: 10.1126/science.373.6550.36
- Zhu, B., Wang, D. and Wei, N. (2022) Enzyme discovery and engineering for sustainable plastic recycling. Trends in Biotechnology. [Online] 40(1), 22-37. doi: 10.1016/j.tibtech.2021.02.008
- Lu, H., Diaz, DJ., Czarnecki, NJ., Zhu, C., Kim, W., Shroff, R., Acosta, DJ., Alexander, BR., Cole, HO., Zhang, Y., Lynd, NA., Ellington, AD. and Alper, HS. (2022) Machine learning-aided engineering of hydrolases for PET depolymerization. Nature. [Online] 604, 662-667. doi: 10.1038/s41586-022-04599-z