It was Charles Darwin himself who helped popularise the study of carnivorous plants, describing them as the “most wonderful plants in the world”1. A number of different carnivorous plants have independently evolved pitcher organs to catch their arthropod prey2, but how do they work and why do they look so similar?
How do Pitcher Plants Demonstrate Convergent Evolution?
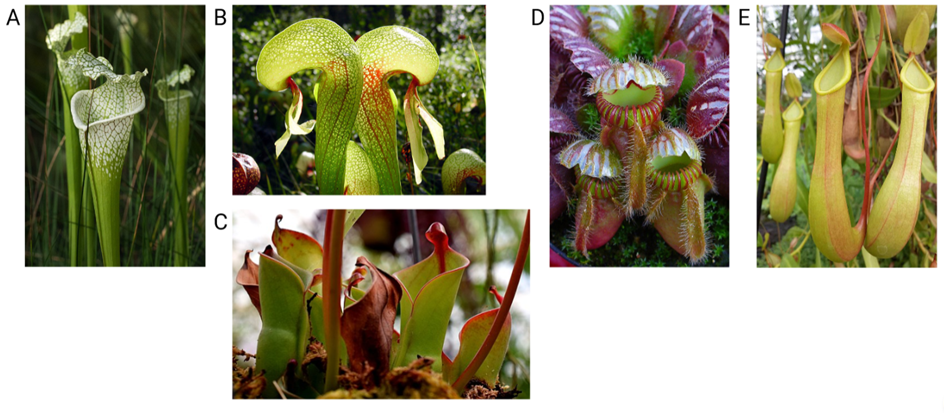
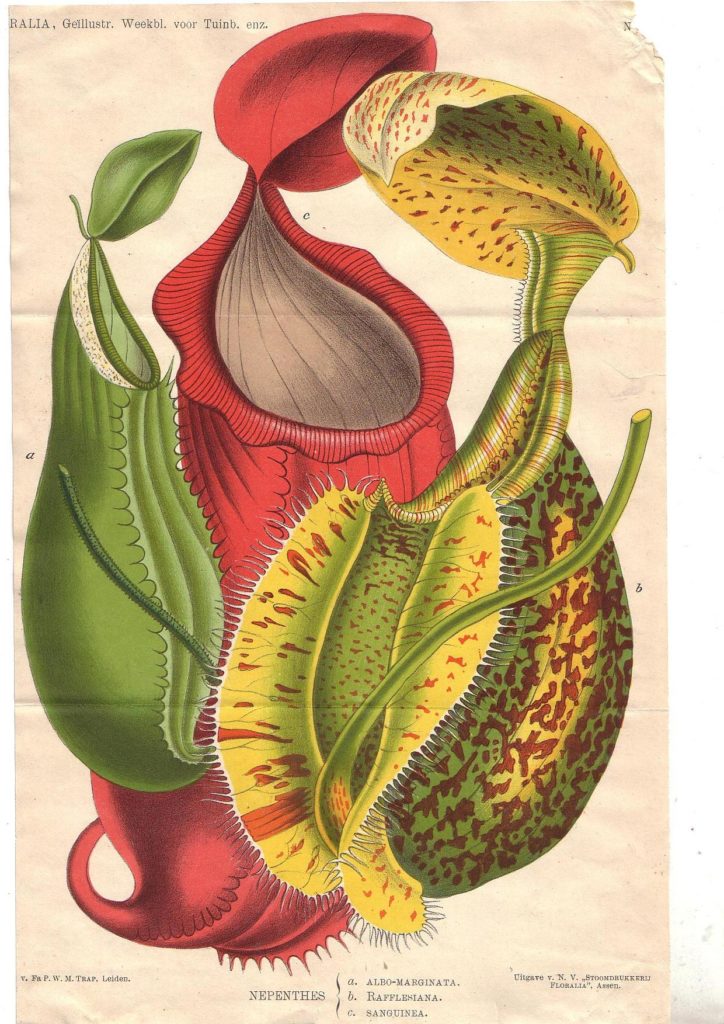
The term ‘pitcher plant’ is used to describe carnivorous eudicot plants that develop similar pitfall traps to attract, trap and digest their prey2. Superficially, the pitcher organs of all five eudicot groups appear very similar (Figure 1) and we might easily assume that these plant groups are closely related. In reality, evolutionary trees constructed using DNA and protein sequences demonstrate that these pitchers have independently evolved in three eudicot plant families: Sarraceniaceae (including Sarracenia spp., the cobra lily and the sun pitchers), Nepenthaceae and Cephalotaceae9,10.
Pitcher plants are therefore a classical example of convergent evolution, the independent evolution of phenotypic (observable trait) similarities between distinct species11. Darwin gives a lucid description of convergent evolution in his Origin of Species:
“As two men have sometimes independently hit on the same invention… it appears that natural selection… has produced similar organs, as far as function is concerned, in distinct organic beings, which owe none of their structure in common to inheritance from a common progenitor” (pp. 238)12.
In other words, the pitcher organs of these three plant families are not the result of common ancestry, but instead reflect independent evolution of the same solution to similar selective pressures.
The Diversity of Pitcher Plants
It is worth first considering the diversity of the plants grouped under the umbrella term ‘pitcher plants’ before we discuss the convergent evolution of their pitcher organs.
Sarracenia (Sarraceniaceae)
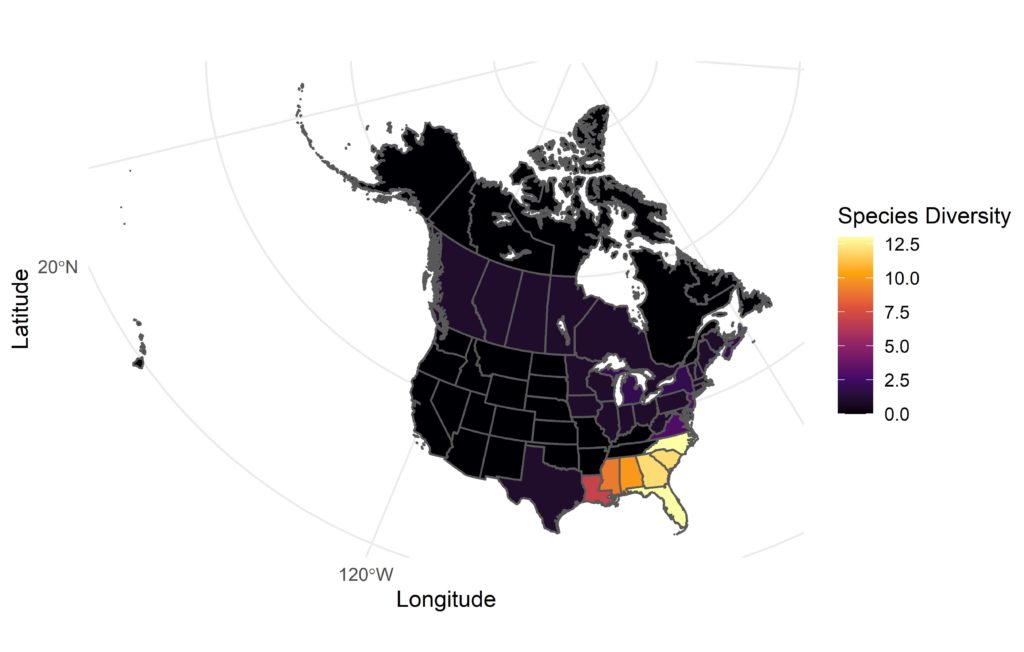
The Sarracenia genus contains a number of temperate pitcher plant species distributed across fens, swamps, coastal and grassland plains across much of North America2. The majority of the Sarracenia species are concentrated into the south-eastern states of the USA, but some species extend their distribution as far as the west coast of Canada (Fig. 2). Species of Sarracenia are easily recognised by their characteristic terrestrial pitchers which have a thin, tall shape growing in clusters from the ground (Fig. 1A).
Darlingtonia californica, the Cobra Lily (Sarraniaceae)
This single species occupies waterlogged boggy soils, often near streams, in the mountainous regions of western USA, especially Sierra Nevada and mountain ranges of Oregon and California2. Its name owes to its cobra-shaped pitchers which appear to be a modified, twisted version of the conventional Sarracenia pitcher (Fig. 1B). The cobra lily is thought to lack most of the digestive enzymes deployed by other pitcher plants and can regulate the level of its digestive fluids using water flow to and from the root system.
Heliamphora, the Sun Pitchers (Sarraniaceae)
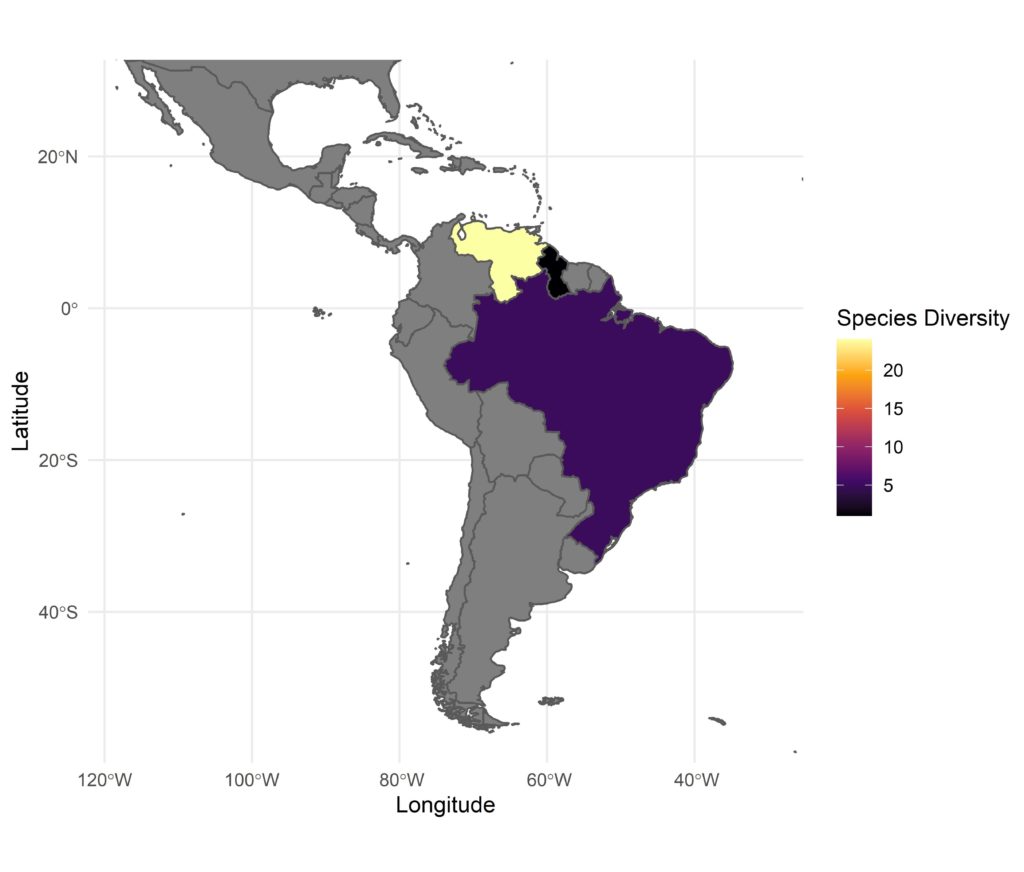
The sun pitchers are a genus of subtropical pitcher plants growing in highland meadows across a relatively wide range in the north-east of South America2 (Fig. 3). Their pitchers also appear to be a variation on the Sarracenia theme but with a more open mouth and a reduced lid known as a nectar spoon, which acts as a lure for prey (Fig. 1C). These species are also thought to lack digestive enzymes but instead relies on commensal pitcher communities for prey digestion.
Cephalotus follicularis, the Albany Pitcher (Cephalotaceae)
The Albany pitcher is the only species in the Cephalotus genus, representing an independent evolutionary origin of plant carnivory2. This species is endemic to the coastal lowlands of south-western Australia. It produces small, terrestrial pitchers in tight clusters that appear similar to a squashed Nepenthes-like form (although not closely-related) with a notably ridged peristome (Fig. 1D).
Nepenthes, the Tropical Pitchers (Nepenthaceae)
Nepenthes is a species-rich tropical pitcher plant genus with a wide distribution across much of southern and south-eastern Asia as well as Australia, the Seychelles and Madagascar2 (Fig. 4). Lowland species grow in humid rainforests at lower altitudes, whereas highland species occupy mountain forests. These species produce both terrestrial and aerial pitchers specialising in capturing ground-dwelling and aerial insects, respectively. The Nepenthes pitchers are more bulbous than those of Sarraniaceae and closely-resemble the conventional pitcher shape (Fig. 1E).
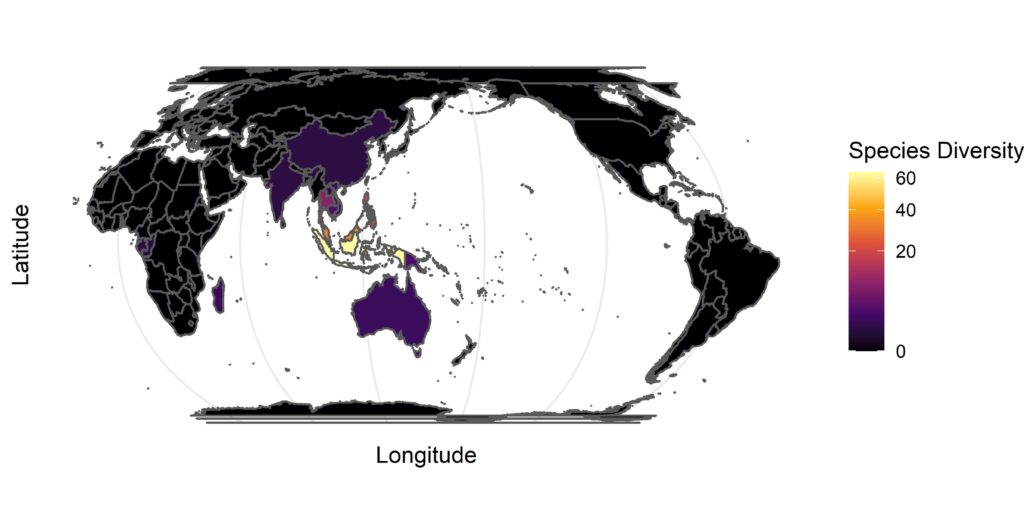
This genus is also notable for some of the only examples of divergence from the usual pitcher-prey ecology amongst pitcher plants. For example, the highland species N. lowii gains 57-100% of its required nitrogen from tree shrew Tupala montana faeces by its aerial pitchers acting as living lavatories14. Similarly, the lowland species N. rafflesiana elongata gains ~1/3 of its nitrogen from the faeces of Hardwicke’s woolly bats (Kerivoula hardwickii hardwickii) which uses its aerial pitchers as a roost during the day15. Some species have also evolved mutualisms to enhance their capture and digestion of arthropod prey. For instance, N. bicalcarata has the peristome of its pitchers regularly cleaned by Camponotus schmitizi ants which occupy its hollow tendrils16. Experimental data suggests that these ant cleaners can restore capture efficiency of pitchers following contamination of the peristome and this may explain why N. bicalcarata has especially long-lived pitchers.
The Pitcher Organ
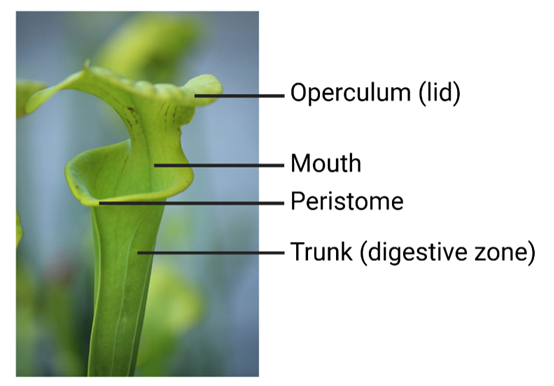
The striking convergence of pitcher plants is best demonstrated by comparing the structure and functions of the pitcher organ between the Sarraceniaceae, Nepenthaceae and Cephalotaceae2,10.
The slippery rim of the pitcher, the peristome (Fig. 2), deploys nectaries and trichomes to attract and trick arthropod prey into falling into the pitcher. Sun pitchers (Sarraceniaceae), Nepenthes spp. (Nepenthaceae), and possibly the Albany pitcher plant (Cephalotaceae), have convergently evolved a particular microtopography of the peristome to maintain this slipperiness10. The parallel ridges on the peristome in these plant groups trap water and ensure that insects walking across the rim aquaplane (slip) into the pitcher for digestion.
Inside the pitchers there is a waxy zone underneath the peristome to prevent arthropods prey from climbing back out. All of the pitcher plant groups have functionally convergent structures to maintain this waxy zone, although there is some variation in the exact mechanisms10. For instance, folds in the cuticle in Nepenthes spp. (Nepenthaceae), the Albany pitcher plant (Cephalotaceae), and sun pitchers (Sarraceniaceae) have independently evolved to reduce the ability of insect claws to grip the waxy zone. Members of the Sarraceniaceae family also use downward-facing hairs and aldehyde crystals extending from the inner wall to force the captured prey downwards towards the digestive fluids.
Finally, all pitchers have a digestive zone below the mouth of the pitcher (Fig. 5) where the prey is digested to release nutrients for the carnivorous plants. There is a stunning level of convergent evolution in the common deployment of digestive enzymes within the digestive fluids of Nepenthes spp. (Nepenthaceae), Sarracenia spp. (Sarraceniaceae) and the Albany pitcher (Cephalotaceae)10. These groups have convergently co-opted these digestive enzymes from stress-responsive genes using, in some positions, the exact same amino acid substitutions in the protein sequences18. This is convergent evolution at a molecular scale (technically, genetic parallelism11). On the other hand, sun pitchers and the cobra lily (Sarraceniaceae) may not produce digestive enzymes but instead rely on commensal organisms occupying their pitcher fluid for digestion10.
There are other aspects of convergence in the structure and function of the pitcher organs in these species. Many species of each of the three pitcher plant families deploy similar visual signals to attract nectar-feeding flying insects into the pitchers, namely: dark UV-absorbing centre of the pitcher with radial striping and dots19. The communities occupying the inside of the pitchers may have also converged between these groups20. Nepenthes spp. (Nepenthaceae) and Sarracenia spp. (Sarraceniaceae) host closely-related and functionally-similar bacteria and eukaryotes within their pitchers. Their microbiomes are enriched in functions for digesting prey such as fatty acid degradation, chitinases (chitin makes up insect exoskeletons and fungal hyphae walls) and amino acid degradation.
Why Have Pitchers Evolved Multiple Times?
The key model used to explain the evolution of carnivorous plants is Givnish’s cost-benefit model of plant carnivory: carnivorous plants can only evolve in bright, nutrient-poor but waterlogged environments where photosynthesis is constrained by nutrients in the soil9. The nutrients obtained from prey must improve the rate of photosynthesis more than the cost of carnivory to photosynthesis. Indeed, carnivory can be very costly in terms of pitcher development and maintenance, and due to the reduced proportion of photosynthetic leaves. More recent work is also focusing on additional trade-offs to plant carnivory, such as the cost-benefits of attracting prey vs. pollinators to the pitcher plants. Overall, these trade-offs mean that carnivorous plants have slower photosynthesis and growth than other plant species in the same environments therefore they mainly colonise extreme habitats (e.g. temperate bogs) where competition for growth is lower. This lifestyle may also depend on the abundance of arthropod prey, explaining why some pitcher plants in prey-limited habitats have diverged in their nutritional strategies for capturing nutrients14.
The repeated, independent evolution of pitchers may therefore be explained as a common solution to the selective pressures imposed by bright, waterlogged environments9. Pitcher organs must be an efficient evolutionary solution to catch terrestrial and aerial insects in these environments and to provide nutrients to these plants surviving in nutrient-poor soils. Other trap morphologies may be too inefficient for prey capture or too costly to develop and maintain.
References
- Thompson, K. (2019) Darwin’s Most Wonderful Plants. 2nd ed. London: Profile Books.
- Krol, E., Plachno, BJ., Adamec, L., Stolarz, M., Dziubinska, H. and Trebacz, K. (2011) Quite a few reasons for calling carnivores ‘the most wonderful plants in the world’. Annals of Botany. [Online] 109(1), 47-64. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3241575/
- Assen, S. (1900) “Nepenthes” Lithograph. [Image Online] Available from: https://commons.wikimedia.org/wiki/File:Nepenthes_Stoomdrukkerij_Floralia.jpg
- Rhododenrites. (2018) Sarracenia leucophylla at the Brooklyn Botanic Garden. Wikipedia Commons. [Image Online] Available from: https://commons.wikimedia.org/wiki/File:Sarracenia_leucophylla_at_the_Brooklyn_Botanic_Garden_(81396).jpg
- NoahEIhardt. (2005) Darlingtonia californica ne1. Wikipedia Commons. [Image Online] Available from: https://commons.wikimedia.org/wiki/File:Darlingtonia_californica_ne1.JPG
- Incidencematrix. (2015) Heliamphora pulchella (23508099002). Wikipedia Commons. [Image Online] Available from: https://commons.wikimedia.org/wiki/File:Heliamphora_Pulchella_(23508099002).jpg
- H. Zell (2009) Cephalotus follicularis 0001. Wikipedia Commons. [Image Online] Available from: https://commons.wikimedia.org/wiki/File:Cephalotus_follicularis_0001.JPG
- Keministi. (2019) Nepenthes alata pitchers. Wikipedia Commons. [Image Online] Available from: https://commons.wikimedia.org/wiki/File:Nepenthes_alata_pitchers.jpg
- Ellison, AM. and Gotelli, NJ. (2001) Evolutionary ecology of carnivorous plants. Trends in Ecology and Evolution. [Online] 16(11), 623-629. Available from: https://www.sciencedirect.com/science/article/pii/S0169534701022698
- Thorogood, CJ., Bauer, U. and Hiscock, SJ. (2017) Convergent and divergent evolution in carnivorous pitcher plant traps. New Phytologist. [Online] 217(3), 1035-1041. Available from: https://nph.onlinelibrary.wiley.com/doi/full/10.1111/nph.14879
- Stern, DL. (2013) The genetic causes of convergent evolution. Nature Reviews Genetics. [Online] 14, 751-764. Available from: https://www.nature.com/articles/nrg3483
- Darwin (1859) The Origin of Species. 2nd ed. Edison, NJ, USA: Castle Books
- Schlauer, J. and Walker, R. (2015) The Carnivorous Plants Database. Encyclopaedia of Life. [Website] Available from: https://opendata.eol.org/ne/dataset/carnivorous-plants-database/resource/6a6bc398-9928-48ac-8754-251556964538
- Clarke, CM., Bauer, U., Lee, CC., Tuen, AA., Rembold, K and Moran, JA. (2009) Tree shrew lavatories: a novel nitrogen sequestration strategy in a tropical pitcher plant. Biology Letters. [Online] 5, 632–635. Available from: https://royalsocietypublishing.org/doi/10.1098/rsbl.2009.0311
- Grafe, TU., Schoner, CR., Kerth, G., Junaidi, A. and Schoner, MG. (2011) A novel resource-service mutualism between bats and pitcher plants. Biology Letters. [Online] 7(3), 436-439. Available from: https://pubmed.ncbi.nlm.nih.gov/21270023/
- Thornham, DG., Smith, JM., Grafe, TU. and Federle, W.(2012) Setting the trap: cleaning behaviour of Camponotus schmitzi ants increases long-term capture efficiency of their pitcher plant host, Nepenthes bicalcarata. Functional Ecology. [Online] 26(1), 11-19. Available from: https://besjournals.onlinelibrary.wiley.com/doi/full/10.1111/j.1365-2435.2011.01937.x
- Rillke. (2019) Sarracenia flava IMG 6043. Wikipedia Commons. [Image Online] Available from: https://commons.wikimedia.org/wiki/File:Sarracenia_flava_IMG_6034.jpg
- Fukushima, K., Fang, X., Alvarez-Ponce, D., Cai, H., Carretero-Paulet, L., Chen, C., Chang, T-H., Farr, KM., Fujita, T., Hiwatashi, Y., Hoshi, Y., Imai, T., Kasahara, M., Librado, P., Mao, L., Mori, H., Nishiyama, T., Nozawa, M., Palfalvi, G., Pollard, ST., Rozas, J., Sanchez-Gracia, A., Sankoff, D., Shibata, TF., Shigenobu, S., Sumikawa, N., Uzawa, T., Xie, M., Cheng, Z., Pollock, DD., Albert, VA., Li, S. and Hasebe, M. (2017) Genome of the pitcher plant Cephalotus reveals genetic changes associated with carnivory. Nature Ecology and Evolution. [Online] 1, 0059. Available from: https://www.nature.com/articles/s41559-016-0059
- Biesmeijer, JC., Giurfa, M., Koedam, D., Potts, SG., Joel, DM. and Dafni, A.(2005) Convergent evolution – floral guides, stingless bee nest entrances and insectivorous pitchers. Naturwissenschaften. [Online] 92(9), 444-450. Available from: https://pubmed.ncbi.nlm.nih.gov/16133103/
- Bittleston, LS., Wolock, CJ., Yahya, BE., Chan, XY., Chan, KG., Pierce, NE. and Pringle, A. (2018) Convergence between the microcosms of Southeast Asian and North American pitcher plants. eLife. [Online] 7, e36741. Available from: https://elifesciences.org/articles/36741